GUID: P-1a971.981 58.86.NV_7370
Phone / Fax:
+375 17 322-22-25
+375 17 322-22-24
+375 17 322-22-26
+375 29 166 50 50 (А1)
+375 29 266 50 50 (МТС)
Our Products
- Antibiotics Tests in Milk
- Inhibitory detection test
- Laboratory equipment
- Centrifuges
- Indicator strips
- Autoclaves
- Scales
- Thermometers
- Packing
- PureTrust ATP monitoring
- ATP monitoring PIONEERPRODUKT CleanTrust
- MICROFAST® substrates
- Nutrient media
- Ice cream sticks
- Consumables
- Detergents and disinfectants
- Treatment agent
- Milk filters
- Wipes
- Gloves
- Sampling
A new GMP inspection procedure for veterinary drug manufacturers will come into force in 2026.

Photo is illustrative in nature. From open sources.
The main change is that instead of two separate procedures (licensing control and submitting an application for confirmation), a single combined inspection will now be conducted. A new certification procedure for pharmaceutical inspectors has also been approved, and they will be included in the register of certified specialists. As a reminder, effective September 1, 2023, RUSSIA introduced a requirement for a GMP certificate of compliance for the sale of veterinary products on the domestic market.
Test for Atnibiotics in Milk
 Rapid tests for determining the residual amount of tetracyclines in meat
Rapid tests for determining the residual amount of tetracyclines in meat ANTIBIOTICS / ELISA TESTS
ANTIBIOTICS / ELISA TESTS Express tests for determining the residual amount of β-lactams, tetracyclines, chloramphenicol, streptomycins in milk, whey
Express tests for determining the residual amount of β-lactams, tetracyclines, chloramphenicol, streptomycins in milk, whey TEST KIT for determination of inhibitory agents PIONEERPRODUKT® DASH-TEST, WC0040
TEST KIT for determination of inhibitory agents PIONEERPRODUKT® DASH-TEST, WC0040 PIONEER MEIZHENG BIO-TECH (5 in1) JC1165 / Rapid tests for the determination of the residual amount of halofuginone, flavomycin, novobiocin, flunixin, dexamethasone / prednisolone in milk, whey
PIONEER MEIZHENG BIO-TECH (5 in1) JC1165 / Rapid tests for the determination of the residual amount of halofuginone, flavomycin, novobiocin, flunixin, dexamethasone / prednisolone in milk, whey PIONEER MEIZHENG BIO-TECH (5 in 1) JC0726 / Rapid tests for determining the residual amount of Bacitracin, ansamycins, clindamycin, spiramycin, florfenicol in milk, whey
PIONEER MEIZHENG BIO-TECH (5 in 1) JC0726 / Rapid tests for determining the residual amount of Bacitracin, ansamycins, clindamycin, spiramycin, florfenicol in milk, whey- Rapid tests for determining the residual amount of chloramphenicol in meat
- Rapid tests for fluoroquinolone, erythromycin, lincomycin, tillosin and tilmycosin residues in milk, whey
- PIONEER MEIZHENG BIO-TECH (5 in1) JC0586 - Antibiotic tests 5 in 1 / Rapid tests for determining the residual amount of β-lactams, tetracyclines and cephalexin in milk, whey
- Rapid 4 in 1 tests for determining the residual amount of neomycin, kanamycin, gentamicin, spectinomycin in milk, whey
Laboratory equipment
 Robotic measuring complex based on milk analyzer "Expert Super Plem"
Robotic measuring complex based on milk analyzer "Expert Super Plem" Bürkle IceSampler
Bürkle IceSampler Mastic Milk Indicator Strips, 100 pcs
Mastic Milk Indicator Strips, 100 pcs IRF-454 B2M refractometer with illumination and additional scale (Russia)
IRF-454 B2M refractometer with illumination and additional scale (Russia) ROTAVISC IKA Viscometer (Germany)
ROTAVISC IKA Viscometer (Germany) IKA PETTE vario single-channel laboratory pipettes with variable volume (Germany)
IKA PETTE vario single-channel laboratory pipettes with variable volume (Germany)- Heating plate Tagler PN-4030MK
- Automated measuring complex "Laktan 1-4M" isp. 700
- Metal closets
- Laboratory heating plate PRN-6050-2
- Tagler TSLM 1-8 milk centrifuge (without heating) (Russia)
- Bürkle BeefSteaker
- OHAUS Pioneer (PR) precision scales 0.001g, (220g, 420g, 520g)
- Indicator strips "NS-Chlorine", 25 pcs.
- Laboratory hotplate Rommelsbacher CT 2203/TC
Packing
 Siliconized paper for hygiene products
Siliconized paper for hygiene products KH PACK® Straight Packing Paper
KH PACK® Straight Packing Paper GableTop aseptic packaging
GableTop aseptic packaging Parchment
Parchment Grease and barrier paper KH PACK®
Grease and barrier paper KH PACK® Paper sacks
Paper sacks- Korreks for desserts
- Laminating paper KH PACK®
- Backed Foil
- Paper for micro-ribbed
- KH PACK® tartlet paper
- Laminating paper KH PACK®
- Korreks for confectionery
- Cartons for milk and dairy products
- Cover
Additional Products
 Pepsin whey pork
Pepsin whey pork J-Bottom technology
J-Bottom technology Ice cream sticks (round)
Ice cream sticks (round) Ice cream sticks Standard 114
Ice cream sticks Standard 114 General purpose environment of SPC "Biocompass-S" (Uglich)
General purpose environment of SPC "Biocompass-S" (Uglich) Ice cream sticks Standard 93
Ice cream sticks Standard 93- Auxiliaries for sugar products
- Ice cream sticks Magnum (curly)
- Ice cream sticks (with logo)
- Wafer cup and cone
- Petri dish 90 mm
Consumables
 Mug for milking the first streams of milk.
Mug for milking the first streams of milk. Pump for artificial ventilation of the lungs
Pump for artificial ventilation of the lungs Non-returnable cup for udder treatment
Non-returnable cup for udder treatment Disposable nitrile gloves (packing 100 pieces)
Disposable nitrile gloves (packing 100 pieces) Veterinary Needles Reusable
Veterinary Needles Reusable Spare pacifier
Spare pacifier- Pre-milking udder cleaner (20 l)
- Disinfectant with washing effect (10kg)
- Milk filters - primary cleaning
- Alkaline detergent (20l / 24kg)
- Veterinary glove to the shoulder and through the neck
- Mug for milking the first streams of milk, lengthwise
- Latex gloves (long cuff with roller), (color blue, pack of 50 pieces)
- Bag filter 32
- Dosing syringe, hose attachment
Microbiology
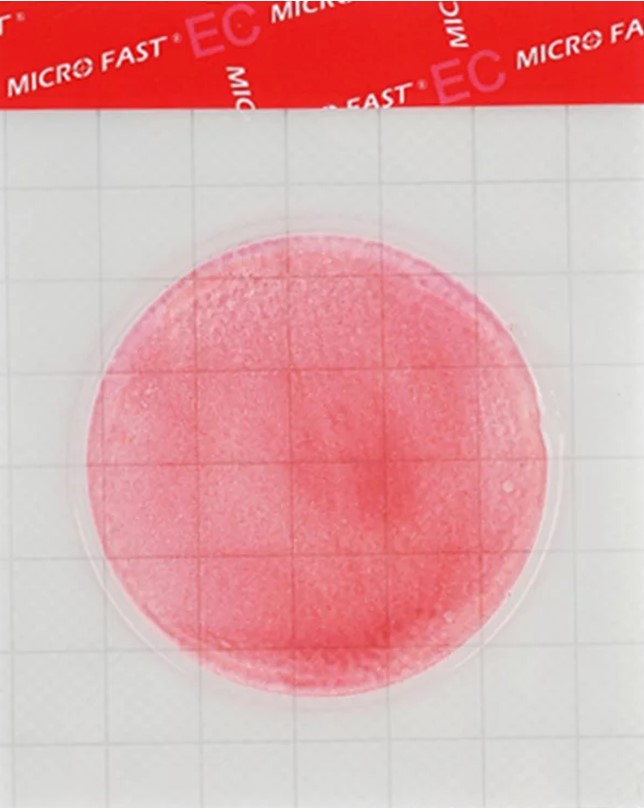 MicroFast® Coliform & E.coli Count Plate
MicroFast® Coliform & E.coli Count Plate MicroFast® Salmonella Count Plate (SAL), for the determination of Salmonella in food and environmental samples (Catalog #LR1006)
MicroFast® Salmonella Count Plate (SAL), for the determination of Salmonella in food and environmental samples (Catalog #LR1006) MicroFast® Bacillus cereus Count Plate (catalog number LR1010)
MicroFast® Bacillus cereus Count Plate (catalog number LR1010) MicroFast® Lactic Acid Bacteria Count Plate (Part Number LR1312)
MicroFast® Lactic Acid Bacteria Count Plate (Part Number LR1312) MicroFast® Environmental Listeria Count Plate
MicroFast® Environmental Listeria Count Plate Substrate for determining the number of staphylococci (Catalog number LR1005) MicroFast® Staphyloccocus aureus Count Plate
Substrate for determining the number of staphylococci (Catalog number LR1005) MicroFast® Staphyloccocus aureus Count Plate- Coliform Count Plate (catalog number LR1002) MicroFast® Coliform Count Plate
- Yeast & Mold Count Plate (cat. no. LR1003) MicroFast® Yeast & Mold Count Plate
- Substrate for accelerated determination of QMAFAnM, (catalog number LR1321)
- MicroFast® Staphyloccocus aureus Confirmation Plate Staph.aureus Confirmation Plate (cat. no. LR1005Q)
- Substrate for determining QMAFAnM (catalog number LR1001)
- MicroFast® Microbiological Substrates
- MicroFast® Enterobacteriaceae Count Plate (cat. no. LR1011)
Our News
 Как дегустаторы "разбирают" вкус сыра и какие этапы проходит каждый образец, рассказала эксперт31.10.2025
Как дегустаторы "разбирают" вкус сыра и какие этапы проходит каждый образец, рассказала эксперт31.10.2025 Лукашенко поручил по-хозяйски развивать территории заповедников и осмотрел "деревню будущего"31.10.2025
Лукашенко поручил по-хозяйски развивать территории заповедников и осмотрел "деревню будущего"31.10.2025 Порядок там, где постоянно работают в тонусе. Сергеенко посетил сельхозпредприятия Браславского района31.10.2025
Порядок там, где постоянно работают в тонусе. Сергеенко посетил сельхозпредприятия Браславского района31.10.2025- Строго придерживаться технологии и болеть за свое дело. Как исключить падеж скота, рассказал председатель СПК31.10.2025
- Как жили дети войны? // Про блюдце замороженного молока, школу, недетские пытки и подвиги30.10.2025
- Минсельхозпрод рассказал, какие белорусские продукты наиболее востребованы в Африке30.10.2025
- В Минской области намолочено 2,5 млн тонн зерна вместе с рапсом и кукурузой29.10.2025
- Personal responsibility, modernization. What measures is the Ministry of Agriculture and Food taking to reduce mortality?29.10.2025
- На БУТБ началась реализация желатина для фармацевтической промышленности29.10.2025
- Проверьте, знаете ли вы историю на уровне ЕГЭ26.10.2025
- Lukashenko spoke out against simple solutions for the Vitebsk region and actions along the path of least resistance. 25.10.2025
- Производство молока, инвестиции. Какие точки роста на пятилетку видит руководство Витебской области25.10.2025
- Первичная задача - дойти до каждого сельхозпредприятия. Депутат о развитии АПК Витебской области25.10.2025
- Совместные проекты и обмен опытом. В каких направлениях Беларусь и Казахстан готовы развивать сотрудничество в АПК 24.10.2025
- Беларусь и Алтайский край намерены увеличить биржевую торговлю сельхозпродукцией24.10.2025
- Минск и Алжир готовят переговоры на высшем уровне. Почему Беларусь нацелилась на Север Африки?24.10.2025
Articles
 ФГБУ «ВГНКИ» получил статус референс-центра ФАО по контролю качества ветеринарных препаратов31.10.2025
ФГБУ «ВГНКИ» получил статус референс-центра ФАО по контролю качества ветеринарных препаратов31.10.2025 Модернизация птицефабрики «Карантайская» в Дагестане: инвестиции составят 945 миллионов рублей31.10.2025
Модернизация птицефабрики «Карантайская» в Дагестане: инвестиции составят 945 миллионов рублей31.10.2025 Обновление ветеринарных правил по борьбе с РРСС свиней вступает в силу в России31.10.2025
Обновление ветеринарных правил по борьбе с РРСС свиней вступает в силу в России31.10.2025- Конфликт между «Автотехцентром» и «Токаревской птицефабрикой»: иск на 18,7 млн рублей31.10.2025
- Аномальная жара в Воронежской области привела к гибели более 90 тысяч птиц31.10.2025
- АГРОСИЛА инвестирует 1,15 млрд рублей в модернизацию Пермской птицефабрики до 2026 года31.10.2025
- Россельхознадзор провел надзорный контроль за 18 тысячами тонн животноводческой продукции в Кировской области31.10.2025
- В III квартале 2025 года возбуждено 32 уголовных дела за нарушения в сфере животноводческой продукции31.10.2025
- На миллион меньше: Аргентина завершит 2025 год с сокращением поголовья крупного рогатого скота третий год подряд31.10.2025
- Уругвай усиливает санитарный контроль, чтобы сохранить лидерство на мировом рынке мяса31.10.2025
- RaboResearch: Мировой рынок говядины будет расти даже в условиях неопределенности31.10.2025
- Дешёвая аргентинская говядина? Нет, лучше открыть границу для мексиканского скота31.10.2025
- Цены на борщевой набор снизились, яйца и мясо подорожали в Тульской области31.10.2025
- В Тюменских кафе выявили нарушения при использовании мяса без ветеринарных документов31.10.2025
- Цены на говядину в Братске возросли более чем на 25%31.10.2025
- Цены на курицу выросли на 30%: новая реальность для семейного бюджета31.10.2025
Economics
 10 reasons to take a deposit04.05.2025
10 reasons to take a deposit04.05.2025 Михельсон предупредил о росте цен на газ в ЕС при отказе от СПГ из России31.10.2025
Михельсон предупредил о росте цен на газ в ЕС при отказе от СПГ из России31.10.2025 Швейцария ввела дополнительные санкции против России и Белоруссии31.10.2025
Швейцария ввела дополнительные санкции против России и Белоруссии31.10.2025- Dmitriev warned the EU of losses following the ban on plumbing fixtures from Russia.31.10.2025
- A tanker carrying Russian oil bound for India has been turned back after US sanctions.30.10.2025
- Минфин США снял санкции с Милорада Додика и членов его семьи30.10.2025
- Уитакер заявил об альтернативных санкциям мерах давления США на Россию29.10.2025
- ЛУКОЙЛ решил продать зарубежные активы из-за санкций29.10.2025
- Орбан решил обсудить с Трампом санкции против России29.10.2025
- Стармер оценил эффект санкций против «Роснефти» и ЛУКОЙЛа для Украины29.10.2025
- Bloomberg рассказал о выжидании индийских НПЗ после санкций против России29.10.2025
- The US has exempted Rosneft's German business from sanctions.29.10.2025
- The NYT sees a new level of US economic warfare in Trump's sanctions.25.10.2025
- The Kremlin promised a response to sanctions in line with Russia's interests.25.10.2025
- Zakharova promised "tough steps" in response to the 19th EU sanctions package.25.10.2025
- США ввели санкции против президента Колумбии25.10.2025
Antibiotics in Milk
 В Британии предупредили о риске для миллионов из-за супербактерий06.01.2025
В Британии предупредили о риске для миллионов из-за супербактерий06.01.2025 Moscow court sides with Indian company in dispute with Health Ministry26.11.2024
Moscow court sides with Indian company in dispute with Health Ministry26.11.2024 Scientists estimate increase in mortality due to drug-resistant bacteria29.10.2024
Scientists estimate increase in mortality due to drug-resistant bacteria29.10.2024- Antibiotics for livestock and pesticides found in poisoned family's home29.10.2024
- Izvestia reported on the shortage of widely used antibiotics in Russia29.10.2024
- The Ministry of Health called data on the shortage of antibiotics unreliable29.10.2024
- Scientists warn of threat of return to pre-penicillin times29.10.2024
- The Ministry of Health explained how attitudes towards antibiotics changed during the pandemic07.05.2024
- WHO explains the risks of taking antibiotics "just in case"06.05.2024
- Doctors warn of bad practices after government decision on antibiotics25.04.2024
- The Ministry of Health removed antibiotics and hormones from the standard treatment of ARVI25.04.2024
- Antibiotics in oil: myth or reality?06.03.2024
- Antibiotics in sour cream: myth or reality?05.03.2024
- Antibiotics in goat milk: effects, problems and control measures16.02.2024
- The Japanese will stop producing the popular antibiotic vilprafen in Russia23.12.2023
- Antibiotics in Milk21.12.2023
Antibiotics in Meat
 Antibiotics in pollock25.02.2024
Antibiotics in pollock25.02.2024 Antibiotics in herring: myth or reality?12.02.2024
Antibiotics in herring: myth or reality?12.02.2024 Antibiotics in perch10.02.2024
Antibiotics in perch10.02.2024- Antibiotics in sprat: facts and myths10.02.2024
- Antibiotics in tuna: an important health and environmental issue09.02.2024
- Antibiotics in meat30.01.2024
- Antibiotics in chebureks: myth or reality?29.01.2024
- Antibiotics in cutlets: problem or myth?18.01.2024
- Antibiotics in Chicken: Where Are the Highest Concentrations?17.01.2024
- Antibiotics in carp17.01.2024
- Where Are More Antibiotics in Chicken: Reality and Cautions16.01.2024
- Antibiotics in Salmon: Safety and Product Quality16.01.2024
- Antibiotics in Turkey15.01.2024
- Antibiotics in Sal: Reality and Safety Issues15.01.2024
- Antibiotics in Fried Dumplings: Facts, Risks and How to Stay Safe15.01.2024
- Antibiotics in sausages14.01.2024
10 Interesting Facts
 Antibiotics in Coffee: Myths and Reality03.05.2025
Antibiotics in Coffee: Myths and Reality03.05.2025 Forged forks: 10 interesting facts16.05.2024
Forged forks: 10 interesting facts16.05.2024 Swimming pool and weight loss: 10 interesting facts10.03.2024
Swimming pool and weight loss: 10 interesting facts10.03.2024- Tests for antibiotics in milk - 10 interesting facts07.03.2024
- Cleaning the kettle from scale, 10 interesting facts...06.03.2024
- Antibiotics in beer: 10 interesting facts04.03.2024
- Wild boar, how to survive...01.03.2024
- Purulent mastitis, 10 interesting facts27.02.2024
- Lemon and alcohol: 10 interesting facts25.02.2024
- Mint - 10 interesting facts25.02.2024
- Wild boar, 10 interesting facts20.02.2024
- Wild boar and domestic pig: comparison and advantages20.02.2024
- Cottage cheese, 10 interesting facts20.02.2024
- 10 Interesting Facts About Milk19.02.2024
- How to Clean a Toilet - 10 Interesting Facts (Acid vs Alkaline)18.02.2024
- Goat's milk: 10 interesting facts16.02.2024
Diseases of cattle
 Dicroceliosis in cattle09.03.2024
Dicroceliosis in cattle09.03.2024 Demodicosis in cattle01.03.2024
Demodicosis in cattle01.03.2024 Purulent mastitis of cattle27.02.2024
Purulent mastitis of cattle27.02.2024- Hypodermatosis in cattle20.02.2024
- Hemonchoz in cattle11.02.2024
- Bursitis in cattle30.01.2024
- Brucellosis in cattle29.01.2024
- Bronchopneumonia in calves27.01.2024
- Bronchitis in cattle26.01.2024
- Mortellaro disease in cattle24.01.2024
- White muscle disease in cattle23.01.2024
- Babesiosis in cattle22.01.2024
- Cattle acidosis20.01.2024
- Arthritis in cattle20.01.2024
- Anaplasmosis in cattle18.01.2024
Misc
 Antibiotics for coughs: when they are needed and when they are not11.02.2024
Antibiotics for coughs: when they are needed and when they are not11.02.2024 Подростки тратили стипендию на алкоголь и курение. Суд лишил их права распоряжаться доходами 31.10.2025
Подростки тратили стипендию на алкоголь и курение. Суд лишил их права распоряжаться доходами 31.10.2025 Как правильно трудоустроиться, чтобы не попасть в список не занятых в экономике, рассказали специалисты31.10.2025
Как правильно трудоустроиться, чтобы не попасть в список не занятых в экономике, рассказали специалисты31.10.2025- В Лепельском районе мать пяти несовершеннолетних детей лишена родительских прав31.10.2025
- Нетрезвого водителя с более 3 промилле помог задержать в Минске неравнодушный гражданин31.10.2025
- Входящий и промежуточный контроль. Как усилят мониторинг трудовой дисциплины в Витебской области 30.10.2025
- Повышенное внимание - безопасности пожилых. В Могилеве обсудили работу по Директиве №130.10.2025
- На трассе в Могилевской области задержали пьяного водителя грузовика30.10.2025
- В Дятловском районе мужчина угрожал брату ножом и поджогом, возбуждено уголовное дело30.10.2025
- Врач: в Беларуси доступны новые методы лечения псориаза29.10.2025
- Более 260 лихачей остановили в выходные на дорогах Гомельской области 29.10.2025
- Около 41 тыс. случаев инсульта ежегодно отмечается в Беларуси 29.10.2025
- Нетрезвый водитель на столичной МКАД выехал на встречку29.10.2025
- Отсутствие ремней безопасности, проезд не по своей полосе. На что обращает внимание ГАИ при проверке маршруток 26.10.2025
- В Минтруда напомнили об основных мерах безопасности во время проведения республиканского субботника 25.10.2025
- In Minsk, an unlicensed BMW driver lost control and crashed into a pole.25.10.2025
Cloud of Tags
Поддержкаминистр сельского хозяйстваAppleДжо БайденИмпортПродукты питанияМужчиныПроизводство мясаРоссельхознадзорГазпромЗависимостьТатарстанНефтьВеликобританияПесковВластиПрокуратураЗаконопроектСШАФранцияКлиматФруктыМясокомбинатРосстатКазахстанЛечениеМинздравЗапретГовядинаЭкономикаИнвестицииБразилияGoogleИталияДеньгиЕврокомиссияБолезньПравительствоMicrosoftКитайСмертьОбщепитУченыеBloombergСырыВеликобританииЗдоровьеАнтибиотиковМясоКРСАЧСВьетнамПольшаСвининаАзербайджанРоссияКредитДетиЗеленскийБизнесСубсидииРодителиЕАЭСБеларусьЖивотноеГовядинуМинскУкраинаВакцинацииАлкогольного опьяненияВирусОнлайнМоскваМясо птицыСпутник vCOVID-19ЦентробанкСанкт-ПетербургАлександр ЛукашенкоРоссиянеРаботаRussiaИммунитетЯйцаМЧСДиректорТАССКолбасыКартофельTelegramУборкаОрганизмФермерПроизводство молокаЕСЕгипетЭкспортРоспотребнадзорКризисМинфинВ китаеКарантинДоходыАвстралияЦеныМаслоReutersРубльПраздникСанкцииПтицефабрикаВостокСельское хозяйствоПродукцияПродовольствиеУрожаяНовостиКоронавирусМолоко2024Крупного рогатого скотаИнфляцияКрупный рогатый скотЗаконБройлерЯпонияИсследованияFacebookДолларВыручкаКофеОтпускСудАлкогольВладимир ПутинПитаниеУзбекистанПодмосковьеУкраинеДмитрий песковПензенской областиЛекарстваКанадаДональд ТрампМинсельхозТребованияНедвижимостьВакцинацияГерманияТурция
Persons
Our Partners
Top 10
Our Test - Pioneer Tests
- Express tests for determining the residual amount of β-lactams, tetracyclines, chloramphenicol, streptomycins in milk, whey
- TEST KIT for determination of inhibitory agents PIONEERPRODUKT® DASH-TEST, WC0040
- PIONEER MEIZHENG BIO-TECH (5 in1) JC0586 - Antibiotic tests 5 in 1 / Rapid tests for determining the residual amount of β-lactams, tetracyclines and cephalexin in milk, whey
- PIONEER MEIZHENG BIO-TECH (5 in1) JC0871/ Rapid tests for the determination of the residual amount of β-lactams, tetracyclines, chloramphenicol, streptomycins, ceftiofur in milk, whey.
- PIONEER MEIZHENG BIO-TECH (5 in1) JC1165 / Rapid tests for the determination of the residual amount of halofuginone, flavomycin, novobiocin, flunixin, dexamethasone / prednisolone in milk, whey
"PionerProdukt" LTD 2015 - 2025 - The quality is higher than the price...

