- Antibiotics Tests in Milk
- Inhibitory detection test
- Laboratory equipment
- Centrifuges
- Indicator strips
- Autoclaves
- Scales
- Thermometers
- Packing
- PureTrust ATP monitoring
- ATP monitoring PIONEERPRODUKT CleanTrust
- MICROFAST® substrates
- Nutrient media
- Ice cream sticks
- Consumables
- Detergents and disinfectants
- Treatment agent
- Milk filters
- Wipes
- Gloves
- Sampling
FSBI "VGNKI" is developing a methodology for identifying the content of an extended list of NSAIDs in livestock products
As part of the research, the specialists of the Federal State Budgetary Institution VGNKI are working on the creation of a sensitive method for determining the content of an extended list of non-steroidal anti-inflammatory drugs (NSAIDs) in livestock products using high-performance liquid chromatography with mass spectrometric detection, based on a new approach to sample preparation.
NSAIDs are a group of drugs that have a pronounced analgesic, antipyretic and anti-inflammatory effect. NVPS have long been used in everyday veterinary practice and have proven to be a reliable tool for combating various animal diseases. Medicines accelerate the process of recovery of sick animals, relieve pain, restore blood vessels, normalize metabolism and oxygen metabolism in affected tissues, and reduce body temperature.
However, if the timing of the removal of these drugs from the body of animals is not observed, their residual amounts can be found in products of animal origin. The ingestion of NSAIDs into the human body can lead to damage to the gastrointestinal mucosa and liver, impaired filtering ability of the kidneys, thrombosis and internal bleeding.
And although in the territory of the Customs Union, according to TR TS 021/2011, the content of NSAIDs in food products is not allowed, the number of positive samples detected in livestock products is increasing every year. In addition, new compounds of the NSAID group have recently been synthesized. Therefore, the existing GOST 32881-2014 needs to be improved in the form of an extension to these connections. However, today, when conducting monitoring studies of livestock products, it is more expedient to apply the QuEChERS principle (“quickly, easily, cheaply, efficiently, reliably and safely”), based on dispersive solid-phase purification of extracts.
The methodology developed at VGNKI will be used as part of the state monitoring of the safety of products of animal origin, in order to protect the HEALTH and well-being of the population, and will also provide reliable control over these compounds when exporting products to the EU and CHINA.
 Rapid tests PIONER 5 in 1 for the determination of sulfonamides, tylosin, tilmicosin, lincomycin, erythromycin, fluoroquinolones
Rapid tests PIONER 5 in 1 for the determination of sulfonamides, tylosin, tilmicosin, lincomycin, erythromycin, fluoroquinolones PIONEER MEIZHENG BIO-TECH (5 in 1) JC0726 / Rapid tests for determining the residual amount of Bacitracin, ansamycins, clindamycin, spiramycin, florfenicol in milk, whey
PIONEER MEIZHENG BIO-TECH (5 in 1) JC0726 / Rapid tests for determining the residual amount of Bacitracin, ansamycins, clindamycin, spiramycin, florfenicol in milk, whey Express tests for determining the residual amount of β-lactams, tetracyclines, chloramphenicol, streptomycins in milk, whey
Express tests for determining the residual amount of β-lactams, tetracyclines, chloramphenicol, streptomycins in milk, whey Rapid tests for determining the residual amount of chloramphenicol in meat
Rapid tests for determining the residual amount of chloramphenicol in meat PIONEER MEIZHENG BIO-TECH (5 in1) JC0586 - Antibiotic tests 5 in 1 / Rapid tests for determining the residual amount of β-lactams, tetracyclines and cephalexin in milk, whey
PIONEER MEIZHENG BIO-TECH (5 in1) JC0586 - Antibiotic tests 5 in 1 / Rapid tests for determining the residual amount of β-lactams, tetracyclines and cephalexin in milk, whey PIONEER MEIZHENG BIO-TECH (5 in1) JC0871/ Rapid tests for the determination of the residual amount of β-lactams, tetracyclines, chloramphenicol, streptomycins, ceftiofur in milk, whey.
PIONEER MEIZHENG BIO-TECH (5 in1) JC0871/ Rapid tests for the determination of the residual amount of β-lactams, tetracyclines, chloramphenicol, streptomycins, ceftiofur in milk, whey.- Express tests for determining the residual amount of β-lactams and tetracyclines in milk, whey
- Rapid 4 in 1 tests for determining the residual amount of neomycin, kanamycin, gentamicin, spectinomycin in milk, whey
- Express-tests PIONER 5 in1 for the determination of thiamphenicol, meloxicam, colistine, trimethoprim, sulfonamides
- Rapid tests for fluoroquinolone, erythromycin, lincomycin, tillosin and tilmycosin residues in milk, whey
 VK-75-01 steam sterilizer upgraded version (Tyumen, Russia)
VK-75-01 steam sterilizer upgraded version (Tyumen, Russia) Memmert drying cabinets UN/UF series (Germany)
Memmert drying cabinets UN/UF series (Germany)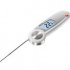 Folding waterproof thermometer Testo 104
Folding waterproof thermometer Testo 104 Heating plate Tagler PN-4030MK
Heating plate Tagler PN-4030MK GP dry-air sterilizer (SKTB, Smolensk)
GP dry-air sterilizer (SKTB, Smolensk) Table for scales BA-CL-0.6 CB (600x400x780)
Table for scales BA-CL-0.6 CB (600x400x780)- Tagler BVR-18 water-reducer bath (Russia)
- Infrared thermometer with laser pointer Testo 830-T1, Testo 830-T2
- LactoScope FT-B - Milk Analyzer with Limited Capabilities for Cream
- Homogenizer T 25 digital ULTRA-TURRAX
- HI 8424 portable pH/ORP meter/thermometer (pH/ORP/T)
- Cylindrical shape weights with head
- testo 206-pH2 - Pocket pH meter
- Heating plate Tagler PN-4030SK
- Electronic thermometer LT-300-H-TC
 Siliconized paper for hygiene products
Siliconized paper for hygiene products KH PACK® tartlet paper
KH PACK® tartlet paper Laminating paper KH PACK®
Laminating paper KH PACK® Parchment
Parchment Ice cream chopsticks
Ice cream chopsticks Korreks for confectionery
Korreks for confectionery- Korreks for desserts
- Grease and barrier paper KH PACK®
- Carton
- KH PACK® bag making paper
- Paper for micro-ribbed
- Cartons for milk and dairy products
- Backed Foil
- Salad dressings
- The paper packing fastened anticorrosive UNIK 14-70 THAT 5453-003-05773103-2005
 Wafer cup and cone
Wafer cup and cone General purpose environment of SPC "Biocompass-S" (Uglich)
General purpose environment of SPC "Biocompass-S" (Uglich) Petri dish 90 mm
Petri dish 90 mm Ice cream sticks Standard 93
Ice cream sticks Standard 93 Ice cream sticks Standard 114
Ice cream sticks Standard 114 Auxiliaries for sugar products
Auxiliaries for sugar products- Ice cream sticks (with logo)
- Ice cream sticks (round)
- J-Bottom technology
- Ice cream sticks Magnum (curly)
- Pepsin whey pork
 Drencher for feeding calves with a flexible probe
Drencher for feeding calves with a flexible probe Anti-catfish milking rings
Anti-catfish milking rings Cassettes for DCC somatic cell counter
Cassettes for DCC somatic cell counter Non-returnable cup for udder treatment
Non-returnable cup for udder treatment Pre-milking udder cleaner (20 l)
Pre-milking udder cleaner (20 l) Cup for disinfection of udder teats (non-returnable)
Cup for disinfection of udder teats (non-returnable)- Acid detergent (20l / 24kg)
- Fall with a loop for cattle
- Napkin reusable for wiping the udder
- Milk bottle
- Liquid soap "Prestige" (yellow, green, red) 5 l
- Foam cup for udder treatment
- Reusable plastic syringe
- Sticky fly trap, 25cm*10m
- Disinfectant with washing effect (10kg)
 MicroFast® Microbiological Substrates
MicroFast® Microbiological Substrates MicroFast® Environmental Listeria Count Plate
MicroFast® Environmental Listeria Count Plate Coliform Count Plate (catalog number LR1002) MicroFast® Coliform Count Plate
Coliform Count Plate (catalog number LR1002) MicroFast® Coliform Count Plate Substrate for accelerated determination of QMAFAnM, (catalog number LR1321)
Substrate for accelerated determination of QMAFAnM, (catalog number LR1321) Substrate for determining the number of staphylococci (Catalog number LR1005) MicroFast® Staphyloccocus aureus Count Plate
Substrate for determining the number of staphylococci (Catalog number LR1005) MicroFast® Staphyloccocus aureus Count Plate MicroFast® Coliform & E.coli Count Plate
MicroFast® Coliform & E.coli Count Plate- MicroFast® Staphyloccocus aureus Confirmation Plate Staph.aureus Confirmation Plate (cat. no. LR1005Q)
- Yeast & Mold Count Plate (cat. no. LR1003) MicroFast® Yeast & Mold Count Plate
- MicroFast® Lactic Acid Bacteria Count Plate (Part Number LR1312)
- MicroFast® Bacillus cereus Count Plate (catalog number LR1010)
- MicroFast® Enterobacteriaceae Count Plate (cat. no. LR1011)
- Substrate for determining QMAFAnM (catalog number LR1001)
- MicroFast® Salmonella Count Plate (SAL), for the determination of Salmonella in food and environmental samples (Catalog #LR1006)
 Belstat reported how much grain, milk, meat, and vegetables are produced in Belarus per capita.14.11.2025
Belstat reported how much grain, milk, meat, and vegetables are produced in Belarus per capita.14.11.2025 "Коллективы АПК способны решать любые задачи даже в непростых условиях". Назаров о заслугах сельхозпроизводителей14.11.2025
"Коллективы АПК способны решать любые задачи даже в непростых условиях". Назаров о заслугах сельхозпроизводителей14.11.2025 "Белорусские продукты - страновой бренд". В Гродно поздравили работников сельского хозяйства области14.11.2025
"Белорусские продукты - страновой бренд". В Гродно поздравили работников сельского хозяйства области14.11.2025- He crawled to the icon with prayer. The true story of a man who overcame drug addiction.14.11.2025
- Лучшие не только по молоку. Какие рекорды поставили в экспериментальной базе имени Котовского13.11.2025
- "Душа радуется, когда видишь результат". Что для аграриев Витебской области важнее наград13.11.2025
- К концу года здесь установят новый рекорд по надоям! В чем секрет успеха ОАО "Великий Двор"13.11.2025
- Как делают колбасу из козлятины, которую оценил Лукашенко? Раскрываем секреты технологического процесса13.11.2025
- Порядок наводят, но нарушения остались. КГК проверил 46 сельхозорганизаций Витебской области 13.11.2025
- Нуга с фруктами, масло с прованскими травами: какие новинки у белорусских предприятий пищепрома 13.11.2025
- Брянская область вошла в число лидеров среди регионов России по товарообороту на БУТБ12.11.2025
- На БУТБ впервые реализовано пюре экзотических фруктов12.11.2025
- Роботизация молочных ферм и ускоренная обработка почвы: что ученые предлагают сельскому хозяйству12.11.2025
- Taking into account the latest advances in animal husbandry, the MTC was officially opened in the Ostrovets District.07.11.2025
- В Беларуси скорректированы ветеринарно-санитарные правила для производителей молочной продукции 07.11.2025
- "Вода лечит, дает силы". Этот белорус строит уникальные лодки 07.11.2025
 Фермеру на Камчатке вынесли предостережение за нарушение правил утилизации биологических отходов14.11.2025
Фермеру на Камчатке вынесли предостережение за нарушение правил утилизации биологических отходов14.11.2025 The Russian Ministry of Agriculture proposes extending veterinary regulations until 2032.14.11.2025
The Russian Ministry of Agriculture proposes extending veterinary regulations until 2032.14.11.2025 Шотландская компания запускает проект Pig Insights для улучшения здоровья свиней с помощью искусственного интеллекта14.11.2025
Шотландская компания запускает проект Pig Insights для улучшения здоровья свиней с помощью искусственного интеллекта14.11.2025- ILPRA представляет трассилер FoodPack 1485 для упаковки свежего мяса птицы в стретч-плёнку14.11.2025
- Банк России ожидает основной эффект от повышения НДС на инфляцию в январе 2026 года14.11.2025
- Новый законопроект об ответственности за утилизацию отходов принят Госдумой в первом чтении14.11.2025
- Потребительские цены: Небольшое увеличение и снижение темпов роста в ноябре14.11.2025
- Рост производства мяса птицы в Казахстане: достижения и перспективы14.11.2025
- Суд Рязани оштрафовал ООО «Жито» за нарушения в контроле качества мяса14.11.2025
- В Красноярске выявили несанкционированную торговлю мясом14.11.2025
- Татарстан планирует создать новую породу гусей14.11.2025
- A fire at the Merci Agro Sakhalin pig farm killed 1,500 pigs, but pork production will not be affected.14.11.2025
- Министр сельского хозяйства предлагает долгосрочные контракты для стабилизации цен на продукты13.11.2025
- Russian agriculture: self-sufficiency continues to grow13.11.2025
- Sustainable growth of the food and processing industries in Bashkortostan13.11.2025
- The State Duma has passed 71 laws to support agriculture starting in 2021.13.11.2025
 10 reasons to take a deposit04.05.2025
10 reasons to take a deposit04.05.2025 The IEA sees a risk of a decline in oil production in Russia due to sanctions.14.11.2025
The IEA sees a risk of a decline in oil production in Russia due to sanctions.14.11.2025 UniCredit заявил о галактических усилиях из-за санкций против России14.11.2025
UniCredit заявил о галактических усилиях из-за санкций против России14.11.2025- США объяснили снятие санкций с проекта АЭС в Венгрии с участием России13.11.2025
- Зеленский ввел санкции против своего бизнес-партнера13.11.2025
- Ukrainian companies have been hit by US sanctions due to their ties to Iran.13.11.2025
- Канада ввела санкции против Игоря Чайки и «Арктик СПГ 2»13.11.2025
- Princess Eugenie's art gallery has been accused of violating sanctions against Russia.13.11.2025
- Reuters узнал, что ЛУКОЙЛ после санкций обратился с просьбой к США13.11.2025
- Рубио завил, что США «особо не на что накладывать санкции» против России13.11.2025
- Британия запретит морские перевозки российского СПГ12.11.2025
- Tokyo called the entry ban on 30 Japanese citizens to Russia unacceptable.12.11.2025
- Kyiv imposed sanctions against Dmitriev and Russia's negotiator in Istanbul.12.11.2025
- Guardian рассказала, как Австралия продолжает получать российскую нефть12.11.2025
- США частично сняли санкции с Сирии12.11.2025
- Bloomberg рассказал о «настоящей драке» в Европе из-за активов ЛУКОЙЛа12.11.2025
 Pharmaceutical companies see a threat to EU security due to bacteria in Ukraine13.11.2025
Pharmaceutical companies see a threat to EU security due to bacteria in Ukraine13.11.2025 В Британии предупредили о риске для миллионов из-за супербактерий06.01.2025
В Британии предупредили о риске для миллионов из-за супербактерий06.01.2025 Moscow court sides with Indian company in dispute with Health Ministry26.11.2024
Moscow court sides with Indian company in dispute with Health Ministry26.11.2024- Scientists estimate increase in mortality due to drug-resistant bacteria29.10.2024
- Antibiotics for livestock and pesticides found in poisoned family's home29.10.2024
- Izvestia reported on the shortage of widely used antibiotics in Russia29.10.2024
- The Ministry of Health called data on the shortage of antibiotics unreliable29.10.2024
- Scientists warn of threat of return to pre-penicillin times29.10.2024
- The Ministry of Health explained how attitudes towards antibiotics changed during the pandemic07.05.2024
- WHO explains the risks of taking antibiotics "just in case"06.05.2024
- Doctors warn of bad practices after government decision on antibiotics25.04.2024
- The Ministry of Health removed antibiotics and hormones from the standard treatment of ARVI25.04.2024
- Antibiotics in oil: myth or reality?06.03.2024
- Antibiotics in sour cream: myth or reality?05.03.2024
- Antibiotics in goat milk: effects, problems and control measures16.02.2024
- The Japanese will stop producing the popular antibiotic vilprafen in Russia23.12.2023
 Antibiotics in pollock25.02.2024
Antibiotics in pollock25.02.2024 Antibiotics in herring: myth or reality?12.02.2024
Antibiotics in herring: myth or reality?12.02.2024 Antibiotics in perch10.02.2024
Antibiotics in perch10.02.2024- Antibiotics in sprat: facts and myths10.02.2024
- Antibiotics in tuna: an important health and environmental issue09.02.2024
- Antibiotics in meat30.01.2024
- Antibiotics in chebureks: myth or reality?29.01.2024
- Antibiotics in cutlets: problem or myth?18.01.2024
- Antibiotics in Chicken: Where Are the Highest Concentrations?17.01.2024
- Antibiotics in carp17.01.2024
- Where Are More Antibiotics in Chicken: Reality and Cautions16.01.2024
- Antibiotics in Salmon: Safety and Product Quality16.01.2024
- Antibiotics in Turkey15.01.2024
- Antibiotics in Sal: Reality and Safety Issues15.01.2024
- Antibiotics in Fried Dumplings: Facts, Risks and How to Stay Safe15.01.2024
- Antibiotics in sausages14.01.2024
 Antibiotics in Coffee: Myths and Reality03.05.2025
Antibiotics in Coffee: Myths and Reality03.05.2025 Forged forks: 10 interesting facts16.05.2024
Forged forks: 10 interesting facts16.05.2024 Swimming pool and weight loss: 10 interesting facts10.03.2024
Swimming pool and weight loss: 10 interesting facts10.03.2024- Tests for antibiotics in milk - 10 interesting facts07.03.2024
- Cleaning the kettle from scale, 10 interesting facts...06.03.2024
- Antibiotics in beer: 10 interesting facts04.03.2024
- Wild boar, how to survive...01.03.2024
- Purulent mastitis, 10 interesting facts27.02.2024
- Lemon and alcohol: 10 interesting facts25.02.2024
- Mint - 10 interesting facts25.02.2024
- Wild boar, 10 interesting facts20.02.2024
- Wild boar and domestic pig: comparison and advantages20.02.2024
- Cottage cheese, 10 interesting facts20.02.2024
- 10 Interesting Facts About Milk19.02.2024
- How to Clean a Toilet - 10 Interesting Facts (Acid vs Alkaline)18.02.2024
- Goat's milk: 10 interesting facts16.02.2024
 Dicroceliosis in cattle09.03.2024
Dicroceliosis in cattle09.03.2024 Demodicosis in cattle01.03.2024
Demodicosis in cattle01.03.2024 Purulent mastitis of cattle27.02.2024
Purulent mastitis of cattle27.02.2024- Hypodermatosis in cattle20.02.2024
- Hemonchoz in cattle11.02.2024
- Bursitis in cattle30.01.2024
- Brucellosis in cattle29.01.2024
- Bronchopneumonia in calves27.01.2024
- Bronchitis in cattle26.01.2024
- Mortellaro disease in cattle24.01.2024
- White muscle disease in cattle23.01.2024
- Babesiosis in cattle22.01.2024
- Cattle acidosis20.01.2024
- Arthritis in cattle20.01.2024
- Anaplasmosis in cattle18.01.2024
 Antibiotics for coughs: when they are needed and when they are not11.02.2024
Antibiotics for coughs: when they are needed and when they are not11.02.2024 Житель Жлобина совращал подростков в ночных переписках14.11.2025
Житель Жлобина совращал подростков в ночных переписках14.11.2025 КОММЕНТАРИЙ: Житель Жлобина совращал подростков в ночных переписках14.11.2025
КОММЕНТАРИЙ: Житель Жлобина совращал подростков в ночных переписках14.11.2025- В Минске трамвай сбил мужчину, он был пьян и шел по путям14.11.2025
- В Горках задержали 18-летнего нетрезвого водителя без прав14.11.2025
- "Сразу это казалось баловством, а потом затянуло". Как наркоман с 15-летним стажем справился с зависимостью13.11.2025
- При наезде грузовика на рынке в Республике Корея погибли 2 человека, 18 пострадали13.11.2025
- Дегустации, конкурсы, дискуссии. Международная выставка-ярмарка PRODEXPO-2025 стартовала в Минске 12.11.2025
- В магазине Витебска грузчик совершил кражу продукции на более Br4 тыс. 12.11.2025
- A drunk driver struck a teenager on a scooter. A court has issued a verdict in the high-profile accident in Gomel.12.11.2025
- МВД выявило 90 продавцов, которые продавали вейпы несовершеннолетним 09.11.2025
- За молитвой и помощью едут даже из дальних стран. Чем уникален Свято-Успенский Жировичский мужской монастырь09.11.2025
- В Беларуси изменены требования к работе автолавок в сельской местности07.11.2025
- В Горках пьяный мужчина повредил четыре автомобиля07.11.2025
- Понятные нормы и баланс интересов. Как планируют усовершенствовать налоговую систему в Беларуси07.11.2025
- С начала года в Брестской области зарегистрировано 26 ДТП по вине нетрезвых водителей 07.11.2025
Persons
Our Partners
Top 10
Our Test - Pioneer Tests
- Express tests for determining the residual amount of β-lactams, tetracyclines, chloramphenicol, streptomycins in milk, whey
- TEST KIT for determination of inhibitory agents PIONEERPRODUKT® DASH-TEST, WC0040
- PIONEER MEIZHENG BIO-TECH (5 in1) JC0586 - Antibiotic tests 5 in 1 / Rapid tests for determining the residual amount of β-lactams, tetracyclines and cephalexin in milk, whey
- PIONEER MEIZHENG BIO-TECH (5 in1) JC0871/ Rapid tests for the determination of the residual amount of β-lactams, tetracyclines, chloramphenicol, streptomycins, ceftiofur in milk, whey.
- PIONEER MEIZHENG BIO-TECH (5 in1) JC1165 / Rapid tests for the determination of the residual amount of halofuginone, flavomycin, novobiocin, flunixin, dexamethasone / prednisolone in milk, whey
